Research
Hematopoiesis
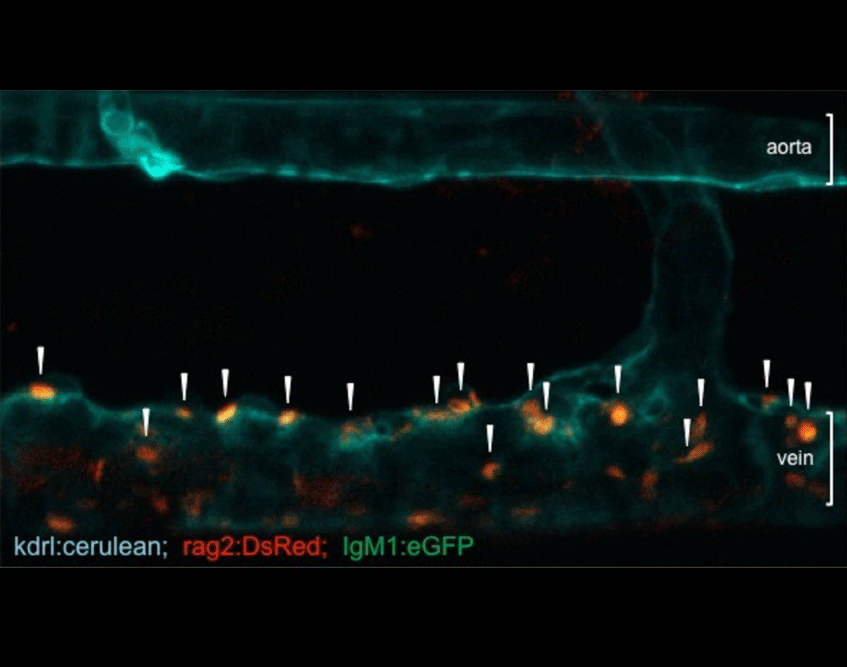
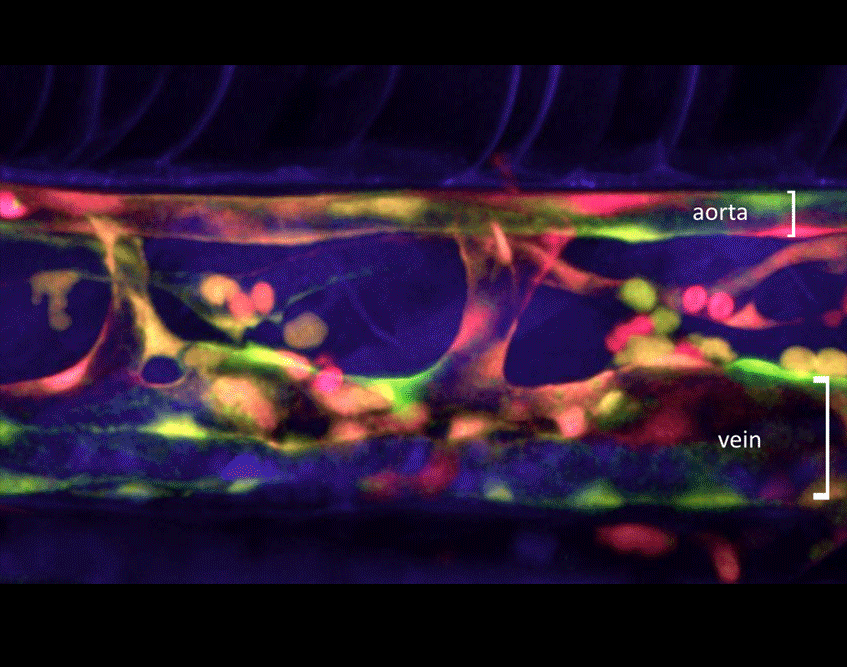
Most of our studies are aimed at understanding how mesodermal derivatives commit to the hematopoietic fates during development. In the vertebrate embryo, hematopoietic cells are specified through four independent waves of precursor production. The first two are termed primitive, in that embryo-specific cell types are born, and include transient waves of erythrocytes and macrophages. The latter two waves produce definitive precursors, defined by their potential to generate multiple lineages of adult hematopoietic cells. Definitive hematopoiesis initiates with the formation of erythromyeloid progenitors (EMPs) in the posterior blood island. While EMPs exist only transiently, they appear to give rise to adult tissue resident macrophages in both the mouse and the zebrafish. Last to appear but most potent are hematopoietic stem cells (HSCs), which maintain homeostasis of the adult blood-forming system. Through confocal timelapse imaging and lineage tracing analyses, we have recently demonstrated that HSCs derive directly from ventral, aortic endothelium during development. Importantly, HSCs appear to similarly derive from this hemogenic endothelium across vertebrate phyla, suggesting that lessons learned in one system will inform others. In this manner, we are working to develop our findings from the zebrafish embryo into improved methodologies to instruct HSC fate in vitro from human pluripotent precursors, a feat that is not currently possible.
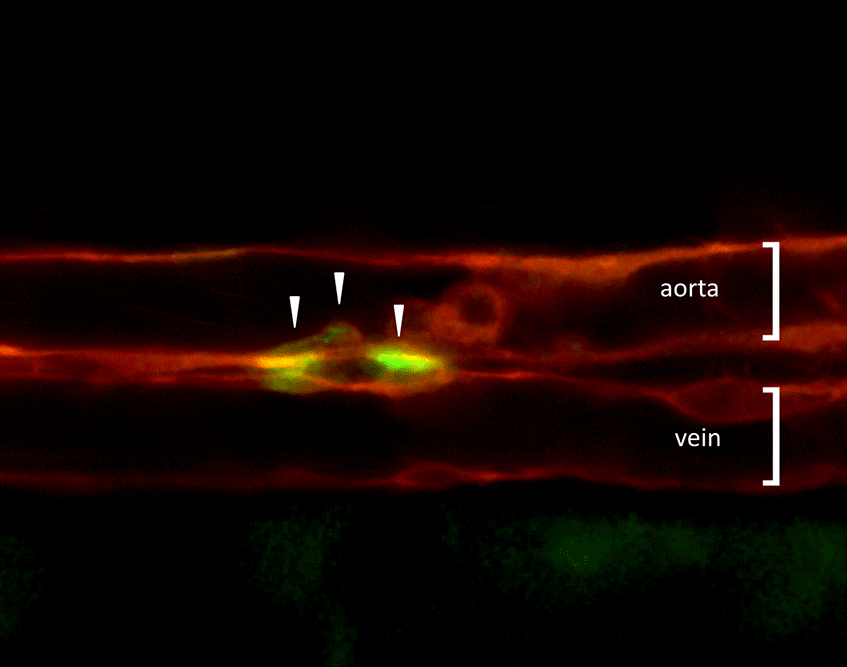
Figure 1. Visualization of HSCs first emerging during development. Combined expression of transgenic reporter lines, which mark either vascular endothelial cells (kdrl:memCherry) or nascent HSCs (gata2b:GAL4; UAS:eGFP), can be utilized to image directly the birth of HSCs (arrowheads) from the aortic floor in the developing zebrafish embryo. A major goal now is to understand the genetic network that regulates this event.
Immunity
We are also interested in understanding the ontogeny of immunity in the zebrafish embryo. Fertilization occurs externally in zebrafish, and the resulting embryos are autonomous from the beginning. Early embryos placed into solutions containing high bacterial titres are extremely resistant to infection. We are examining the components of this early immunity, with the goals of identifying the effectors of innate immunity and developing models of bacterial, protozoan, and helminth infection. We have identified dendritic cells in the zebrafish, and are working to determine when and where antigen presentation occurs to prime the adaptive immune response. We have also discovered that microglia, the resident phagocytes of the brain, are seeded by two different waves of developmental precursors. How these seeding events occur, and how each population may differ in function remain active topics in the laboratory.
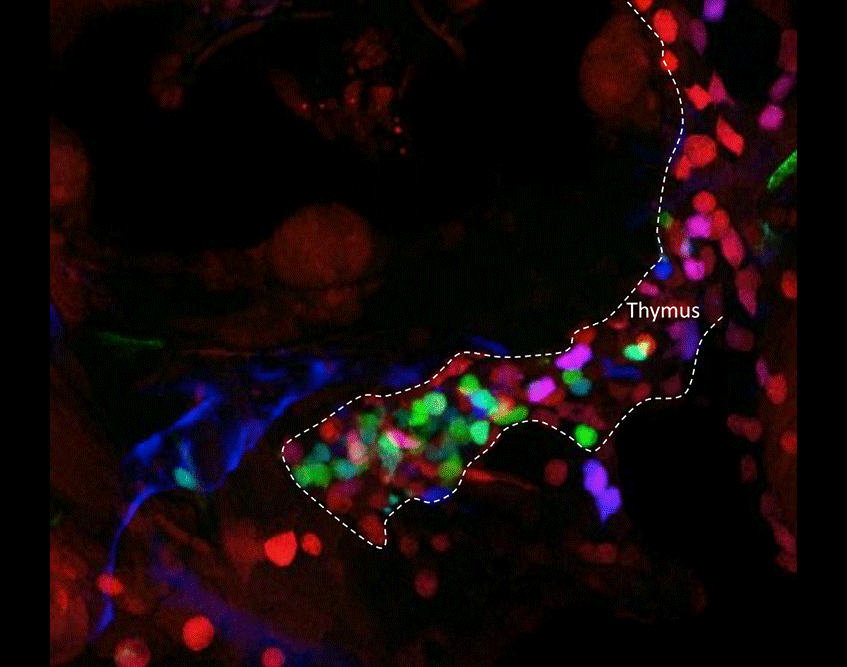
Figure 3. Tracing the output of different HSC clones as they contribute to developing T lymphocytes in the thymus. Outlined with the white dashed line is a thymic lobe that contains thymocytes expressing different fluorophore combinations. Before HSC emergence from the aortic floor, Cre was transiently induced in hemogenic endothelium via a kdrl:cre-ERT transgene. The Cre recombinase then randomly recombined fluorescent transgenes in the ubi:zebrabow construct. Since all developing thymocytes are the progeny of HSCs, the number and diversity of contributing HSCs can be measured by their thymic output.